Our dental infection control solution covers the processes: cleaning, drying, packing, sealing, sterilization and storage, providing a one-stop solution for your infection control project.

*We respect your confidentiality and all information are protected.

















15 years of expertise in airborne infection control and autoclave technology


Certified machines designed to deliver promised results



Comprehensive solutions for all your infection control requirements



Committed to persistently setting the benchmark in infection control

Infection Control Solutions in endoscope reprocessing encompass strategies and technologies to prevent healthcare-associated infections (HAIs) through effective cleaning and disinfection. Key tools include Medical Cleaning Machines like Automated Endoscope Reprocessors (AERs) that automate high-level disinfection, Medical Pouch Sealers to securely package instruments for sterilization, Medical Autoclaves for sterilizing equipment with high-pressure steam, and Medical Storage Containers to maintain sterility during storage. These solutions, supported by disinfectants, training, and monitoring systems, ensure compliance with standards like those from the CDC, minimizing infection risks from complex medical devices like endoscopes.
In today's healthcare environment, preventing healthcare-associated infections (HAIs) is more critical than ever. Effective infection control solutions help safeguard patients, staff, and visitors by minimizing the spread of bacteria, viruses, fungi, and other pathogens. JIAJING MEDICAL, a leading provider with over 15 years of expertise in airborne infection control and autoclave technology, offers comprehensive, one-stop solutions designed to meet the highest standards of safety and efficiency. This guide explores key infection control categories, technologies, and best practices, drawing from advanced systems that comply with global guidelines like ISO, AAMI, FDA, EPA, CDC, and WHO.
Healthcare facilities—hospitals, dental clinics, laboratories, and outpatient centers—face constant risks from airborne and surface contaminants. Proper infection control reduces HAIs, ensures equipment sterility, and maintains regulatory compliance. Modern solutions integrate cleaning, disinfection, sterilization, packaging, and storage to create a seamless workflow in Central Sterile Services Departments (CSSD) and beyond.
Endoscopes require meticulous handling to prevent cross-contamination. Comprehensive systems include:
· Endoscope Cleaning Solutions: Enzyme-based formulas effectively remove biofilms and organic residues.
· Endoscope Storage Cabinets: Feature HEPA-filtered airflow and antimicrobial surfaces, complying with AAMI and SGNA standards for safe, dry storage.
· Deionized Water Systems: Provide pure water for final rinsing.
· Transport and Gas Supply Solutions: Maintain sterility during movement and include detectors for safe gas management.
These tools ensure endoscopes remain contamination-free throughout their lifecycle.
Airborne pathogens are a major threat in enclosed spaces. Advanced air sanitizers include:
· UVC Air Purifiers: Utilize ultraviolet light to eliminate 99.97% of airborne pathogens, aligned with ASHRAE and CDC standards.
· Plasma Air Purifiers: Employ plasma technology to neutralize contaminants.
· Duct-Mounted and HEPA Filter Systems: High-efficiency filtration for installed or portable use.
· Laminar Flow Canopies: Create controlled, clean airflow zones in operating rooms or procedure areas.
These devices are ideal for wards, operating theaters, and clinics to maintain superior indoor air quality.
Thorough cleaning and drying prevent residue buildup:
· Hydrogen Peroxide Surface Disinfectors: Deliver high-level chemical disinfection for surfaces and instruments.
· Ultrasonic Cleaners: Available in volumes from 5L to 100L for precise cleaning.
· Drying Solutions: Include Medical Instruments Vacuum Dryers (for heat-sensitive items), Forced Air Dry Ovens, and Large-Volume Medical Drying Cabinets (400L–600L).
These ensure instruments are spotless and dry before sterilization.
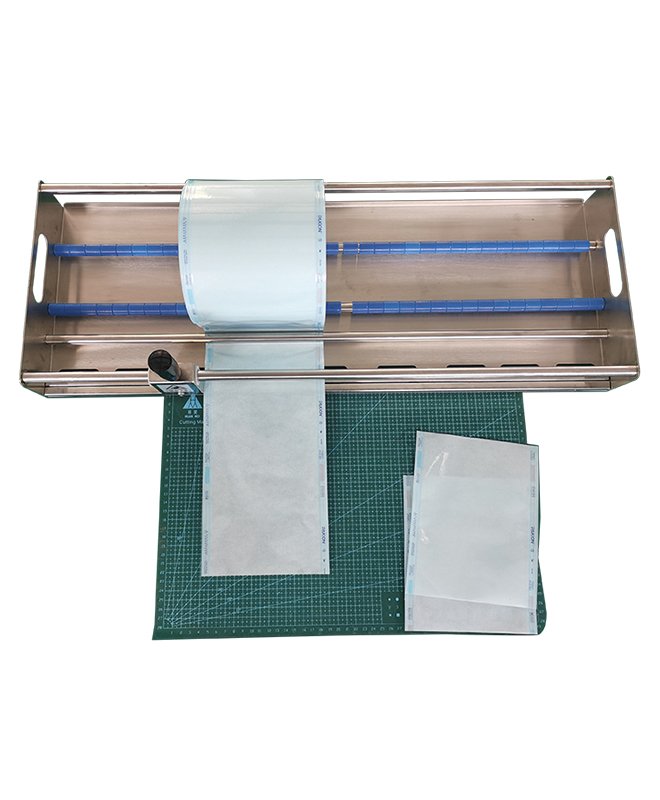
Proper packaging maintains sterility post-processing:
· Rotary Heat Sealers: Automatic continuous models, including variants with double-line printers and built-in printing.
· Cut, Seal, and Print Machines: From semi-automatic to fully automatic (e.g., HCRseal-100).
· Accessory Tools: Sterilization reel cutters, holders, manual sealers, customizable bench-top roll-holders, sliding tables, and CSSD packing tables.
These efficient tools support high-volume workflows with precise sealing.
The core of infection control:
· Class B Benchtop Autoclaves: Available in 23L–45L capacities, with electric or standard doors. These pre-vacuum steam sterilizers meet stringent Class B standards for hollow and porous loads.
Reliable sterilization eliminates even the most resistant microorganisms.
Post-sterilization storage must prevent recontamination:
· Large-Volume Storage Cabinets: Incorporate UVC, ozone, and high-temperature disinfection for ongoing protection.
These cabinets provide secure, long-term sterility maintenance.
· Pathogen Elimination: Advanced technologies (UV, plasma, HEPA, hydrogen peroxide) target bacteria, viruses, and fungi effectively.
· Compliance and Safety: Products adhere to international standards, with rigorous testing and regulatory support.
· Efficiency: One-stop solutions streamline processes, reducing manual errors and turnaround times.
· Versatility: Suitable for medical, dental, and laboratory settings, including biosafety containment.
Look for experience, certifications, and a full-range portfolio. Providers like JIAJING MEDICAL stand out with 15 years of specialized expertise, certified equipment, and a commitment to innovation—setting benchmarks in infection control while offering tailored support.
By implementing robust infection control solutions, healthcare facilities can significantly reduce risks, enhance patient outcomes, and operate with confidence. For customized advice or products, consult certified specialists to align with your facility's needs.
*We respect your confidentiality and all information are protected.