As a leading manufacturer of medical equipment and infection control solutions, our Endoscope Cleaning Solution is a high-performance, enzyme-based formula specifically designed to effectively remove organic residues, proteins, and biofilms from endoscopes during reprocessing. This solution ensures thorough cleaning without damaging delicate instruments, reducing the risk of cross-contamination and supporting compliance with stringent infection prevention standards. Trusted by healthcare professionals worldwide, it delivers superior efficacy, ease of use, and compatibility with automated washers for optimal endoscope hygiene and patient safety.

*We respect your confidentiality and all information are protected.


15 years of expertise in airborne infection control and autoclave technology


Certified machines designed to deliver promised results



Comprehensive solutions for all your infection control requirements



Committed to persistently setting the benchmark in infection control

Our products are designed to meet stringent international and regional standards, including ISO, AAMI, FDA, EPA, CDC, and WHO guidelines. Each product, from our Endoscope Reprocessing Products to our Medical Autoclave and Air Disinfection Machines, undergoes rigorous testing and validation to ensure compliance with the highest standards for infection control and medical device safety. We also provide comprehensive regulatory support to facilitate seamless market entry.
Our solutions, such as the UVC Air Purifier, Plasma Air Purifier, Hydrogen Peroxide Surface Disinfector, and Endoscope Reprocessing Solutions, are engineered to eliminate bacteria, viruses, and fungi. By leveraging advanced technologies like ultraviolet light, low-temperature sterilization, and high-efficiency filtration, chemical high level disinfection etc. our products reduce airborne and surface contamination, significantly lowering the risk of HAIs in healthcare settings.
Our Endoscope Reprocessing Products utilize enzyme-based formulas, chemical disinfectants, and automated cleaning cycles to thoroughly remove biofilms and organic residues, preventing cross-contamination. The Endoscope Storage Cabinet and Transport System feature HEPA-filtered airflow and antimicrobial surfaces, ensuring sterility throughout reprocessing and transit, in compliance with AAMI and SGNA standards.
Our air purification products, including the UVC Air Purifier, Plasma Air Purifier, and Duct-mounted Air Purifier, are ideal for some operating rooms, wards, outpatient clinics, and laboratories. These systems employ HEPA filtration, UV sterilization, and plasma technology to remove 99.97% of airborne pathogens and particulates, ensuring clean and safe air quality in line with ASHRAE and CDC standards.
Our Medical Autoclave uses high-temperature steam for rapid sterilization of heat-resistant instruments. In contrast, our low-temperature sterilization technologies, such as the Hydrogen Peroxide Surface Disinfector, utilize ethylene oxide or hydrogen peroxide , ideal for heat-sensitive devices like endoscopes, ensuring effective sterilization while preserving instrument integrity.
Manual cleaning stands as the cornerstone—and arguably the most critical—step in endoscope reprocessing. Residual organic soils, bioburden, or microbial contaminants on the device can shield pathogens from subsequent high-level disinfection (HLD) or liquid chemical sterilization (LCS), potentially compromising patient safety. Complex flexible and semi-rigid endoscopes feature narrow lumens, intricate channels, valves, elevators, and grooves where blood, proteins, fats, carbohydrates, mucus, and tissue residues can accumulate and dry, forming biofilms that resist penetration by disinfectants.
Effective removal of these residues is essential to enable thorough contact during HLD or LCS, ensuring the endoscope is safe for the next patient. Inadequate cleaning has been linked to outbreaks of multidrug-resistant organisms, underscoring the need for meticulous adherence to protocols.
This comprehensive guide covers:
1. Endoscope Cleaning Standards and Guidelines
2. Point-of-Use Treatment of Endoscopes
3. Manual Leak Testing Procedures for Endoscopes
4. How Endoscopes Are Manually Cleaned (including chemistries, tools, and sinks)
5. Common Mistakes Made During Manual Cleaning
6. Validated Automated Cleaning Alternatives
7. The Broader Importance of Rigorous Cleaning in Flexible Endoscope Reprocessing
For a full overview of all reprocessing steps (including HLD/LCS, drying, and storage), refer to additional resources.
Endoscope reprocessing is governed by rigorous standards to mitigate infection risks. Key documents include:
· ANSI/AAMI ST91: Flexible and semi-rigid endoscope processing in healthcare facilities — This comprehensive standard details workflow, emphasizing manual cleaning's pivotal role in removing bioburden before disinfection/sterilization.
· Association of periOperative Registered Nurses (AORN) — "Guideline for Processing Flexible Endoscopes" highlights complex, validated processes for achieving effective cleaning, HLD, or sterilization.
· Society of Gastroenterology Nurses and Associates (SGNA) — "Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes" provides in-depth protocols for point-of-use treatment, manual cleaning, and beyond. SGNA explicitly states: "[Manual Cleaning] is the most critical step in removing the microbial burden from an endoscope. It requires focused and deliberate attention."
Additional guidance comes from organizations like the Centers for Disease Control and Prevention (CDC), Association for the Advancement of Medical Instrumentation (AAMI), and international bodies such as the European Society of Gastrointestinal Endoscopy (ESGE).
Paramount above all is strict adherence to the validated manufacturer's Instructions for Use (IFUs) for each specific endoscope model. These device-specific, FDA-cleared instructions outline precise steps, compatible chemistries, brushing requirements, flushing volumes, and contact times—essential for validation and regulatory compliance.
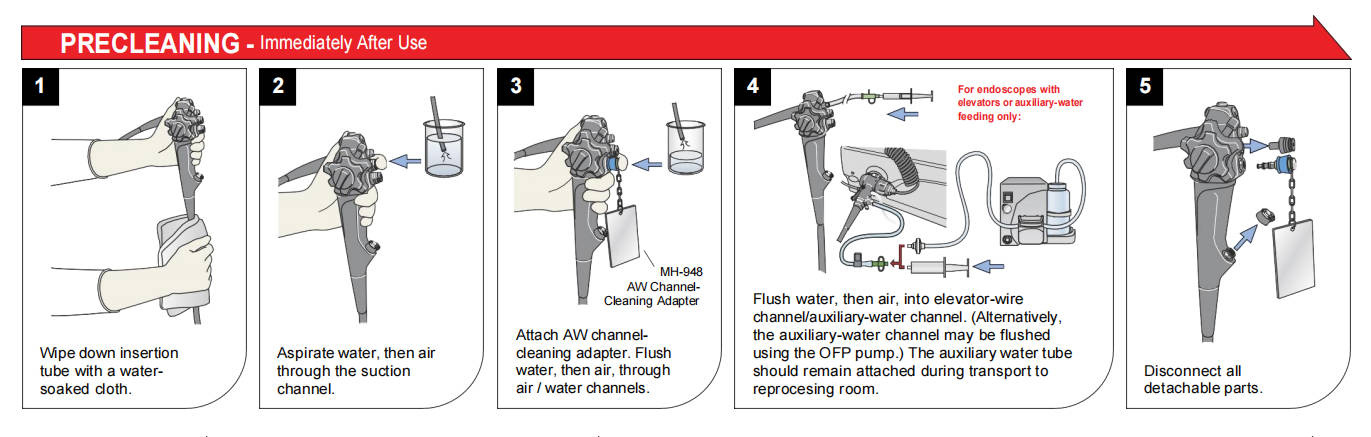
Reprocessing begins immediately in the procedure room with point-of-use (bedside) treatment. This initial step prevents drying of bioburden, which hardens residues and complicates later removal.
Technicians use dedicated kits like INTERCEPT™ Foam or the Revital-Ox™ Bedside Complete Pre-Cleaning Kit to wipe external surfaces, flush channels with enzymatic or detergent solutions, and aspirate debris. Products such as PRE-KLENZ™ Soak Shield initiate enzymatic pretreatment while protecting the distal tip during transport to the decontamination area.
Timely point-of-use treatment (ideally within minutes post-procedure) significantly enhances manual cleaning efficacy, reduces biofilm risk, and aligns with standards mandating immediate pre-cleaning to maintain moist conditions.
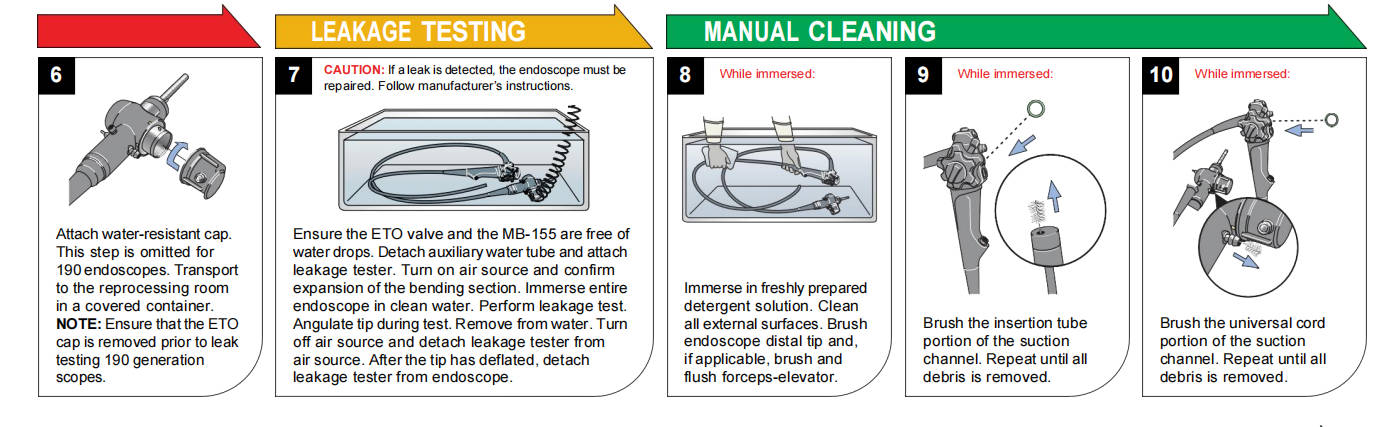
Prior to immersion cleaning, perform manual leak testing per the manufacturer's IFU to detect breaches in the endoscope's integrity. Fluid invasion through cracks or perforations can damage internal components (e.g., imaging sensors, fiberoptics, angulation wires) and foster hidden contamination.
Standard procedure:
1. Attach the leak tester and waterproof cap (if required).
2. Conduct dry leak testing (pressurization without submersion) to identify pinholes.
3. Submerge the insertion tube and observe for continuous bubbles indicating leaks.
4. Fully submerge the entire endoscope, manipulating controls while monitoring for bubbles.
If leaks are detected, quarantine the device for repair. Routine leak testing after every use extends endoscope lifespan and prevents costly internal damage.
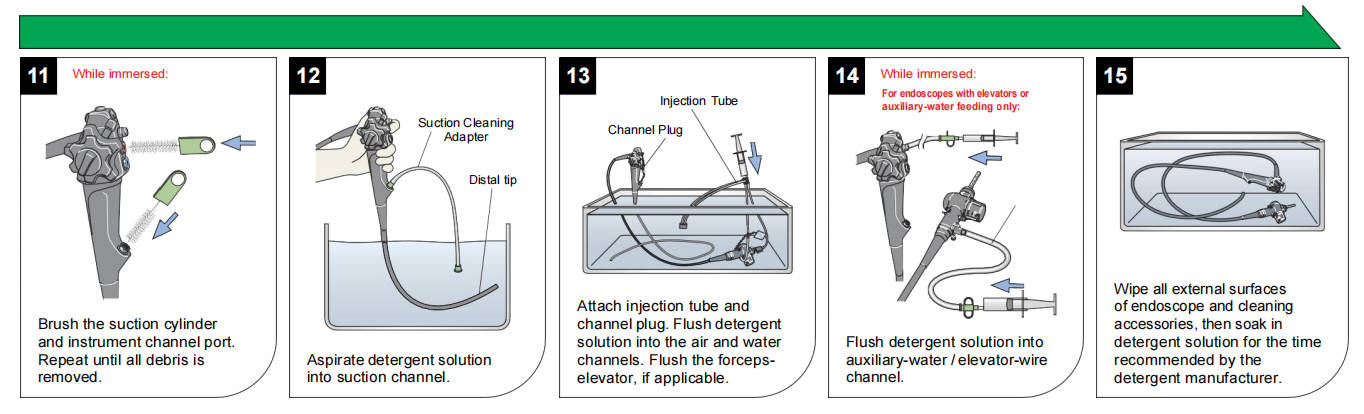
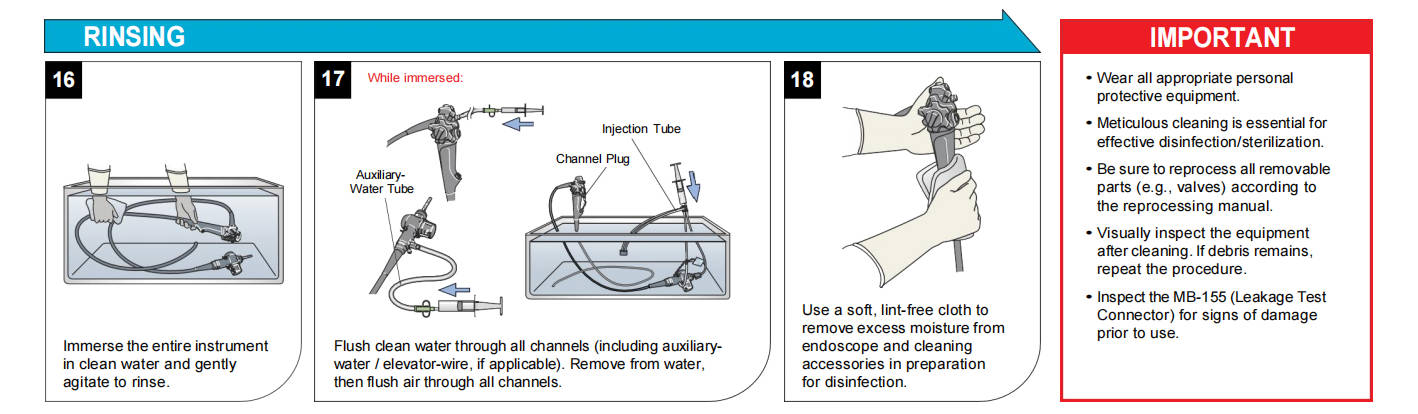
Manual cleaning follows the manufacturer's IFU precisely and may exceed 100 steps for complex devices like duodenoscopes with elevator channels.
Core process for flexible endoscopes:
1. Prepare a multi-basin sink with fresh, low-foaming, pH-neutral, endoscope-specific detergent.
2. Immerse the endoscope fully (with protective caps).
3. Wipe exterior surfaces submerged using dedicated sponges.
4. Detach and clean valves, ports, and removable parts.
5. Brush all accessible channels (suction, biopsy, air/water, elevator, auxiliary) multiple passes with correctly sized, single-use or validated reusable brushes until no visible debris emerges.
6. Clean brushes between passes.
7. Attach model-specific adapters and flush/detergent-irrigate all channels with specified volumes.
8. Soak for the required contact time.
Auxiliary tools like SCOPE BUDDY™ PLUS Endoscope Flushing Aid automate flushing, ensure consistent volumes, and time brushing cycles for compliance.
· Enzymatic Cleaners: Target proteins (proteases), fats (lipases), and carbohydrates (amylases). Effective against blood, mucus, and simethicone residues (e.g., Revital-Ox Enzymatic Detergents).
· Neutral Detergents with Surfactants (e.g., INTERCEPT™ Detergent): Lower surface tension for penetration; non-corrosive to delicate materials; prevent soil redeposition.
Combine for optimal results—enzymatics for breakdown, surfactants for lifting.
· Brushes: Channel-specific diameters; single-use preferred to avoid cross-contamination.
· Sponges: Enzymatic-impregnated for enhanced soil breakdown in crevices; material-compatible to prevent scratching.
These tools mechanically disrupt biofilms, remove debris, and maintain channel patency.
Dedicated reprocessing sinks are recommended over utility sinks. ANSI/AAMI ST91 specifies:
· Ideally three basins: leak testing (water only), cleaning (detergent), rinsing (critical water).
· Alternatives: two-basin or single deep basin with workflow adaptations.
Key features: Sufficient depth/width to avoid tight coiling (preventing damage to internal bundles); ergonomic height-adjustability; integrated flushing ports; hands-free operation for infection control.
1. Skipping Point-of-Use Treatment
2. Inadequate Manual Leak Testing
3. Improper Use of Brushes and Sponges
4. Delayed Reprocessing (>1 hour requires extended protocols)
5. Insufficient Rinsing
6. Improper Handling and Storage (e.g., tight coiling)
7. Neglecting Visual Inspection
8. Ignoring Ergonomics
9. Inadequate Documentation
10. Overlooking Manufacturer's Instructions
Mitigation through training, checklists, and competency assessments is vital.
Manual processes are prone to variability. Most traditional AERs focus on disinfection without validated cleaning.
Robust cleaning protocols—from bedside to advanced automation—are foundational to infection prevention, regulatory compliance, device longevity, and operational efficiency. Investment in staff training, quality tools, and validated technologies safeguards patients and strengthens healthcare outcomes.
Explore JIAJING Medical Point-of-Use Treatment and Manual Cleaning Products. Learn about the Carin-500 / 600 Automated Endoscope Reprocessor the pioneering AER with validated cleaning and LCS claims.
*We respect your confidentiality and all information are protected.