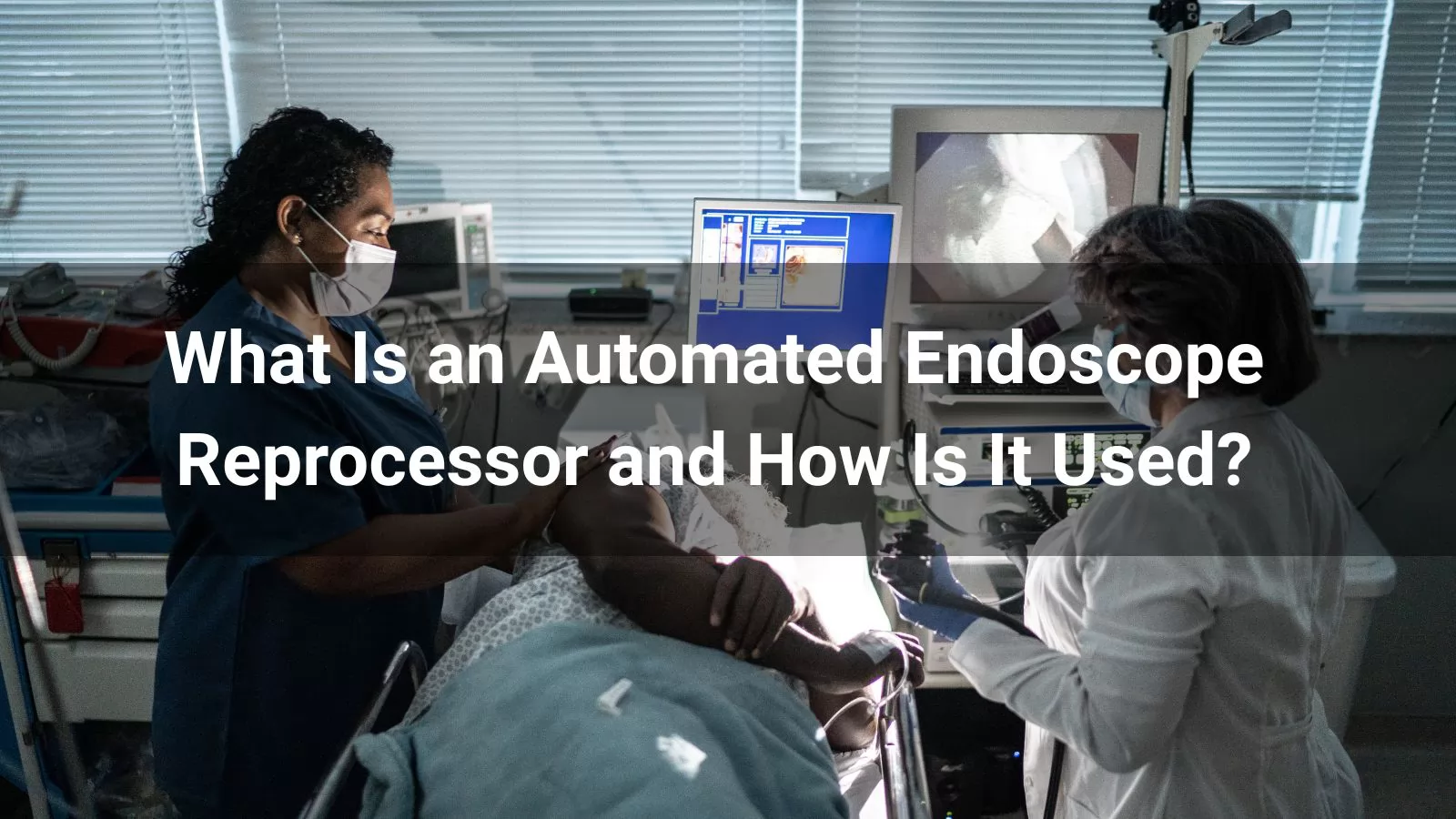
In the rapidly evolving field of minimally invasive procedures, endoscopes play a crucial role in diagnostics and treatments worldwide. With over 250 million endoscopies performed globally each year, ensuring these reusable devices are thoroughly cleaned and disinfected is paramount to patient safety. This is where automated endoscope reprocessors (AERs) come into play. An AER is a specialized medical device designed to automate the cleaning, high-level disinfection (HLD), and sometimes sterilization of flexible and rigid endoscopes, reducing the risk of cross-contamination and human error compared to manual methods.

Unlike manual soaking, which relies heavily on technician precision, AERs standardize the reprocessing workflow by exposing the endoscope's external surfaces and internal channels to high-level disinfectants or liquid chemical sterilants. This process eliminates all viable microorganisms except for small numbers of bacterial spores, aligning with guidelines from organizations like the Association for the Advancement of Medical Instrumentation (AAMI) and the Society of Gastroenterology Nurses and Associates (SGNA). Jiajing Medical, a leading manufacturer in medical device innovation, produces AERs that integrate seamlessly into healthcare facilities, emphasizing efficiency, compliance, and safety.
Endoscope reprocessing is a multi-step process that begins immediately after use to prevent soil drying, which can compromise later stages. The six essential steps—point-of-use treatment, leak testing, manual cleaning and verification, HLD or liquid chemical sterilization (LCS), drying and storage, and transportation—form the backbone of safe reuse. AERs primarily enhance the HLD or LCS phase, providing redundancy to manual cleaning and ensuring consistent results.

Flexible Endoscope Manual Reprocessing Sinks/Basins Workstation
According to ANSI/AAMI ST91:2021, semi-critical devices like endoscopes, which contact mucous membranes or non-intact skin, require thorough cleaning followed by sterilization if possible, or HLD at minimum. AERs achieve this by automating exposure to chemical agents, minimizing staff exposure to hazardous disinfectants and standardizing cycle times. For instance, Jiajing Medical's AER models are engineered for heat-sensitive devices, supporting validated protocols that detect leaks, flush channels, and monitor disinfection efficacy in real-time.
Jiajing Medical's automated endoscope reprocessors operate through a controlled, multi-phase cycle tailored to endoscope complexity. Here's a breakdown of their core functionality:
These features not only comply with FDA evaluations for AER efficacy but also support traceability through integrated software, logging cycle data for audits.

Adopting Jiajing Medical AERs offers several advantages in high-volume endoscopy units:
While AERs excel in disinfection, they complement the entire process. Post-AER, endoscopes undergo drying, storage in aerated cabinets, and secure transport in covered containers to the procedure room. Hand hygiene and protective handling are non-negotiable. Jiajing Medical provides comprehensive solutions, from detergents to cabinets, ensuring end-to-end compliance.
Automated endoscope reprocessors like those from Jiajing Medical are indispensable for modern healthcare, transforming a labor-intensive, error-prone task into a reliable safeguard against infections. By automating critical disinfection steps within a rigorous six-step protocol, they uphold the highest standards of patient care. As endoscopy demand surges, investing in quality AERs isn't just about compliance—it's about delivering optimal outcomes safely and efficiently. For facilities seeking robust, innovative reprocessing, Jiajing Medical stands out as a trusted partner in infection prevention.















*We respect your confidentiality and all information are protected.

This comprehensive guide delves into the technical details, applications, pros and cons, and cost considerations of UVC and Plasma air purifiers to help you make informed decisions for your indoor air quality management needs.

Endoscope reprocessing is a critical healthcare procedure to ensure reusable flexible and rigid endoscopes are safe for patient use by thoroughly cleaning, disinfecting, and/or sterilizing them to eliminate pathogens and prevent infections.

This updated list of the top 10 manufacturers specializes in air sanitizer machines, ranked based on production scale, technological expertise, market presence, export reach, and innovation in disinfection features. The selections draw from industry insights, company histories, and product portfolios as of late 2026.